What is Fusion?

Without fusion, there would be no life on Earth.
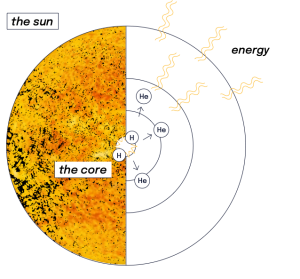
What we see as light and feel as warmth is the result of a fusion reaction in the core of our Sun: hydrogen nuclei collide, fuse into heavier helium atoms and release tremendous amounts of energy in the process.
Over billions of years, the gravitational forces at play in the universe have caused the hydrogen clouds of the early universe to gather into massive stellar bodies. In the extreme density and temperature of the stars, including our Sun, fusion occurs.
How does fusion produce energy?
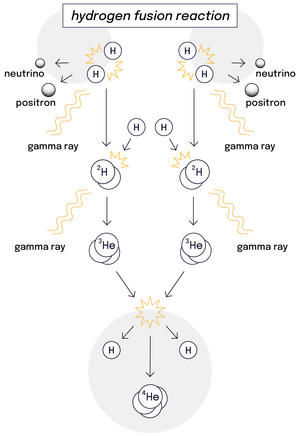
Atoms never rest: the hotter they are, the faster they move. In the Sun's core where temperatures reach 15,000,000 °C, hydrogen atoms are in a constant state of agitation. As they collide at very high speeds, the natural electrostatic repulsion that exists between the positive charges of their nuclei is overcome and the atoms fuse. The fusion of light hydrogen atoms produces a heavier element, helium.
The mass of the resulting helium atom is not the exact sum of the initial atoms, however—some mass has been lost and great amounts of energy have been gained. This is what Einstein's famous formula E=mc² describes: the tiny bit of lost mass (m), multiplied by the square of the speed of light (c²), results in a very large figure (E), which is the amount of energy created by a fusion reaction.
Every second, our Sun turns 600 million tonnes of hydrogen into helium, releasing an enormous amount of energy. But without the benefit of gravitational forces at work in our universe, achieving fusion on Earth has required a different approach.
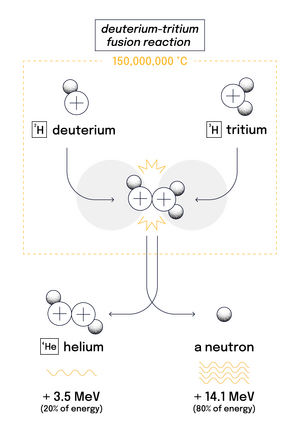
Twentieth-century fusion science identified the most efficient fusion reaction in the laboratory setting to be the reaction between two hydrogen (H) isotopes deuterium (D) and tritium (T). The DT fusion reaction produces the highest energy gain at the "lowest" temperatures. It requires nonetheless temperatures of 150,000,000 degrees Celsius—ten times higher than the hydrogen reaction occurring in the Sun.
Read more about making it work in the laboratory here.