Extracting this deuterium from seawater is a simple and well proven industrial process. "Heavy water", or D2O (water in which deuterium substitutes for hydrogen), is first separated from regular water by chemical exchange processes, and is then submitted to electrolysis in order to obtain deuterium gas.
The market for deuterium is small: it is used in electronics as a replacement for hydrogen in certain industrial processes; in biochemistry as a non-radioactive tracer; in "deuterium arc lamps" for spectroscopy; and, of course, in fusion research.
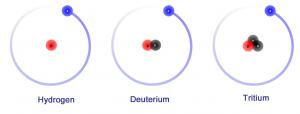
Of all possible fusion reactions, the one that involves deuterium (D) and the other heavy isotope of hydrogen tritium (T) is the "easiest" to achieve in the present state of technology. Despite some downsides—such as the production of highly energetic neutrons, and the fact that tritium is a slightly radioactive element—the DT reaction will probably remain for a long time to come the only way to produce viable fusion energy.
To date, only JET and the American tokamak TFTR have burned the "actual fusion fuels" (deuterium and tritium) and produced significant levels of fusion energy. Present tokamaks or stellarators all conduct their experiments with "deuterium-only" plasmas, whose behaviour in terms of confinement, heating, and general "plasma engineering" is very close to that of a DT plasma.
At Tore Supra, on the other side of the CEA fence, experiments with deuterium plasmas have been ongoing for the past twenty-two years. The installation consumes an average of 3 kilos of deuterium per year, delivered to Cadarache in the form of 50 litre tanks at a pressure of 200 bars (~ 10 cubic metres at ambient pressure). Deuterium is bought from a commercial company and costs around EUR 4,000 per kilo.
"Contrary to many countries, and contrary also to the IAEA, France considers deuterium as a nuclear material," explains François Saint-Laurent, a fusion physicist at CEA's Research Institute on Magnetic Fusion (IRFM) and one of the five Tore Supra pilots. "This implies a very strict follow-up of the quantities of deuterium that we store and use in the machine."