
Of all liquids, water is second only to ammonia in its heat absorption capacity. Its performance, availability and low cost make it the coolant of choice for industrial facilities.
At the mention of "pure water," visions of sparkling mountain springs tend to appear before our mind's eye. But even in its purest natural state, water is far from being pure in a chemical sense—that is, far from containing atoms of hydrogen and oxygen only. An excellent solvent, water almost always contains dissolved minerals, salts and metals, as well as sediments and other impurities.
In ITER, where 20,000 cubic metres of water from the nearby Canal de Provence will be held in the cooling tower basins, and more than 3,000 cubic metres of purified water within ITER's cooling water systems, these extra constituents all add up to one thing—trouble.
"Dissolved salts, minerals or metals in water—what we call ionic impurities—significantly increase the electrical conductivity of water," explains Babulal Gopalapillai, Cooling Water Chemistry Engineer. "Whereas on the contrary, along with its cooling function, water should serve as an electrical insulator in our cooling loops for safety reasons—especially in the cooling loops that service high-voltage electrical systems at ITER."
Impurities can also corrode steel cooling water pipes. "Not only does corrosion translate into downtime and increased maintenance costs," says Babulal, "but the combination of corrosion products in the cooling stream and neutron radiation from the plasma results in the formation of radioactive isotopes." These "activated corrosion products" need to be kept to acceptably low levels in ITER. That's where water treatment at ITER will enter the equation.

Babulal Gopalapillai, ITER's Cooling Water Chemistry Engineer.
Of the 15 cooling loops planned to remove the heat of the ITER installation, more than half are dedicated to cooling the most demanding "clients": the plasma-facing components and the systems that require the purest de-ionized, low-conductivity water. These circuits make up the ITER primary cooling loops, where heat is transferred from its primary source.
Secondary cooling loops, which receive the heat from the primary loops and other sources, have slightly lower requirements in purity. Tertiary loops circulate untreated or "raw" water to cooling towers, from which heat from the secondary loops is ultimately transferred to the environment.
"By segregating the loops according to client requirements, we are able to ensure the purity and safety in the primary loops, avoiding the inadvertent release of radioactive effluents into the environment, while using lower-cost methods elsewhere," explains Babulal.
Purity in the primary loops is ensured by on-line water treatment systems, such as the chemical and volume control systems (CVCS). In a continuous manner, two to five percent of primary loop cooling water circulates through CVCS ion exchange columns and filter units that absorb ionic impurities and filter particles, and keep conductivity and activated corrosion products low.
The CVCS also controls water chemistry, providing regular measurements of conductivity levels, pH and dissolved gases. Chemists at ITER will have a wide array of tools to adjust and control chemical parameters. Are the oxygen molecules dissociating under the influence of radiation? Add hydrogen. Is too much dissolved oxygen in the cooling stream creating oxidation? Add hydrazine.
The secondary loops will also have on-line measurement of pH and conductivity levels. To keep the general level of ionic impurities low, small quantities of water from these loops will be drained when their quality level deteriorates beyond preset limits, and de-ionized water fed back into the loop—a process that Babulal refers to as "feed and bleed."
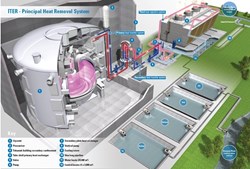
The ITER installation will be actively cooled by ultrapure water circulating in a closed loop for each group of components.
Grab sampling will be used in all loops to cross check the accuracy of online monitoring systems and to test for impurities such as calcium, zinc, sodium, silica, copper, iron, chloride, carbonates, sulphates and phosphates that could contribute to greater corrosivity or conductivity in the water supply. In primary and secondary loops, which are closed systems, water levels will be monitored continuously and any loss due to leakages made up using de-ionized water.
In the open tertiary loops, water levels in the cooling tower basins will be maintained by a continuous supply of makeup water from the Canal de Provence. This makeup flow is needed to compensate for evaporation losses and the regular discharge of water used to control the deterioration in water quality caused by evaporation. Anti-scalant will be added to combat the raw water hardness, and a biocide (ozone) used to prevent the growth of dangerous bacteria.
"The control of water chemistry has been demonstrated to have a significant influence on materials performance," emphasizes Babulal. "During ITER operation, we'll have a chemical laboratory on site, and a chemist on duty during every operating shift." Work is currently ongoing to define the exact chemical specifications required to meet client requirements, and to establish the design of the treatment systems and the sizing of purification systems. "The challenges of ITER lie in the volume of water to be managed in the cooling water systems, and in the diversity of the clients' needs," says Babulal.
And one last thing ...
ITER chemists will have the responsibility of ensuring that water discharged from the ITER cooling water systems meets the strictest environmental standards before release into the cooling water discharge basins and, ultimately, the Durance River. Temperature, pH and chemical parameters will come under close scrutiny in the ITER laboratory and again at the CEA, which is responsible for the water's final release.
The last step in the process doesn't have much to do with chemistry or laboratories, however. Regulations stipulate that local species of fish be introduced into the cooling water basins and observed for six hours. "In the end, after all of our sophisticated means," observes Babulal, "Mother Nature will have the final word!"




